Accelerating Medical Innovation
To Enhance Patient Wellbeing
Biomimetic Innovations

OsStic® Technology was developed to enhance the bodies’ natural healing process using biomimetic agents. OsStic® technology is currently being studied in both soft and hard tissue applications in human and veterinary indications to address current surgical limitations.
OsStic® Technology was developed to enhance the bodies’ natural healing process using biomimetic agents. OsStic® technology is currently being studied in both soft and hard tissue applications in human and veterinary indications to address current surgical limitations.
Watch the video below to learn more about the ‘Magic of OsStic® ‘
Watch the video below to learn more about the ‘Magic of OsStic® ‘
Based on extensive in-vivo studies, OsStic® offers trauma, spinal and dental clinicians and their patients, the opportunity to benefit from the disruptive attributes of this technology through:
Based on extensive in-vivo studies, OsStic® offers trauma, spinal and dental clinicians and their patients, the opportunity to benefit from the disruptive attributes of this technology through:
- Enhanced structural stability
- Faster remodeling into new bone
- Immediate implant stabilization in poor bone quality
- A biological approach to provisional fixation
- Adhesive properties in a wet environment
- Promoting earlier patient mobilization
- Enhanced structural stability
- Faster remodeling into new bone
- Immediate implant stabilization in poor bone quality
- A biological approach to provisional fixation
- Adhesive properties in a wet environment
- Promoting earlier patient mobilization
In 2023, the FDA awarded OsStic® ‘Breakthrough Device’ designation.
In 2023, the FDA awarded OsStic® ‘Breakthrough Device’ designation.
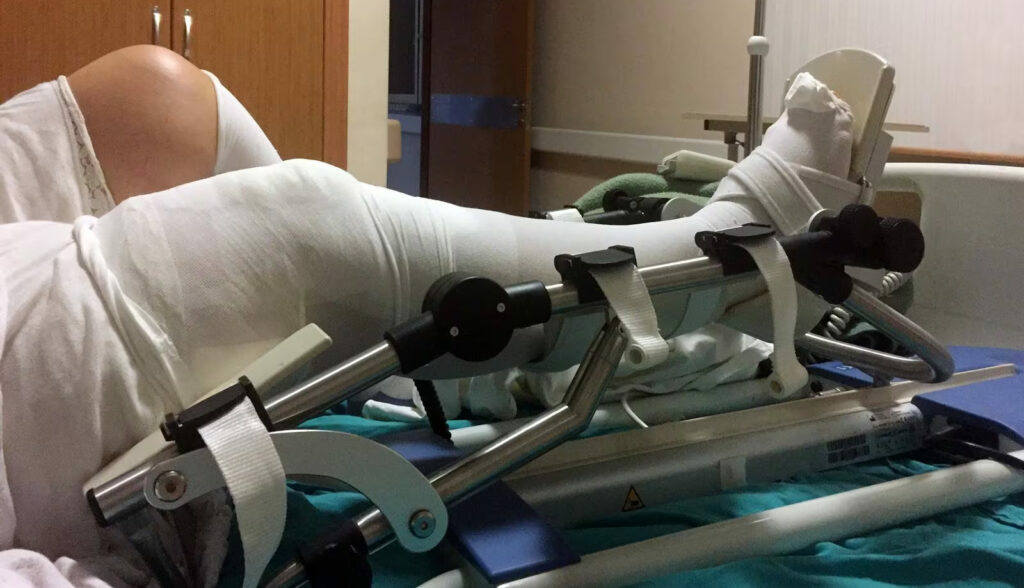
Breakthrough Designation Trauma indication statement:
“OsStic® Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation, or void filling of peri-articular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.” the FDA awarded OsStic® ‘Breakthrough Technology’ designation.
Breakthrough Designation Trauma indication statement:
“OsStic® Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation, or void filling of peri-articular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.” the FDA awarded OsStic® ‘Breakthrough Technology’ designation.
Click here to read the full Press Release
Click here to read the full Press Release
Click here to stay up to date on LinkedIn
Click here to contact us via email or else message us directly at info@pbcbiomed.com
Click here to stay up to date on LinkedIn
Click here to contact us via email or else message us directly at info@pbcbiomed.com

Biomimetic Innovations Ltd is an affiliate of PBC Biomed; a medical device company involved in design, development and manufacturing headquartered in Shannon, Ireland and with offices in Memphis, Tennessee and Chamonix, France.
Biomimetic Innovations Ltd is an affiliate of PBC Biomed; a medical device company involved in design, development and manufacturing headquartered in Shannon, Ireland and with offices in Memphis, Tennessee and Chamonix, France.
Biomimetic Innovations Ltd is dedicated to accelerating medical innovation through the development of a new bone adhesive technology based on biology called OsStic®. The company is the result of a decade long collaboration between GP Bio Ltd and PBC Biomed Ltd, both with extensive expertise in the area of biomaterials and bone repair. Our aim is to commercialise OsStic® in a multitude of applications as a biomimetic adhesive for skeletal and soft tissue repair.
Biomimetic Innovations Ltd is dedicated to accelerating medical innovation through the development of a new bone adhesive technology based on biology called OsStic®. The company is the result of a decade long collaboration between GP Bio Ltd and PBC Biomed Ltd, both with extensive expertise in the area of biomaterials and bone repair. Our aim is to commercialise OsStic® in a multitude of applications as a biomimetic adhesive for skeletal and soft tissue repair.